- For Print
- September 12, 2022
Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today results from a post hoc analysis of three randomized, pivotal, Phase 3 studies (EMBRACE trial/Study 305, Study 301 and Study304) evaluating the efficacy of eribulin mesylate (marketed as HALAVEN®) versus other chemotherapies (Treatment of Physician’s Choice [TPC], capecitabine, and vinorelbine, respectively) in patients living with metastatic breast cancer (mBC) whose tumors have low or no HER2 expression. These data were presented as a poster (Presentation: #259P) at the European Society for Medical Oncology (ESMO) Annual Meeting (#ESMO22), held virtually and in-person in Paris, France from September 9-13, 2022.
The HER2-low breast cancer subtype is a newly defined subset consisting of tumors that would have previously been considered HER2-negative based on an immunohistochemistry (IHC) assay and an in situ hybridization (ISH) assay. HER2-low tumors express low amounts of the HER2 protein, but not enough to be considered HER2-positive. HER2-low is defined as an IHC of 1+ or 2 with a negative ISH. Of the approximate 288,000 new cases of female breast cancer expected to be diagnosed in the U.S. in 2022,1 it is estimated that approximately 80-85% of patients would previously have been considered to have the HER2-negative subtype. Of those patients, about 60% would now be considered to have the HER2-low subtype.2
“In this post-hoc analysis, the outcomes seen in mBC patients whose tumors are considered HER2-low are consistent with the results of the three pivotal Phase 3 clinical trials,” said Dr. Takashi Owa, Chief Scientific Officer, Senior Vice President, Eisai Co., Ltd. “As the oncology community’s understanding of mBC continues to evolve, it’s important that we continue to evaluate the role of existing therapies in new contexts to contribute to the body of knowledge that is available to health care professionals.”
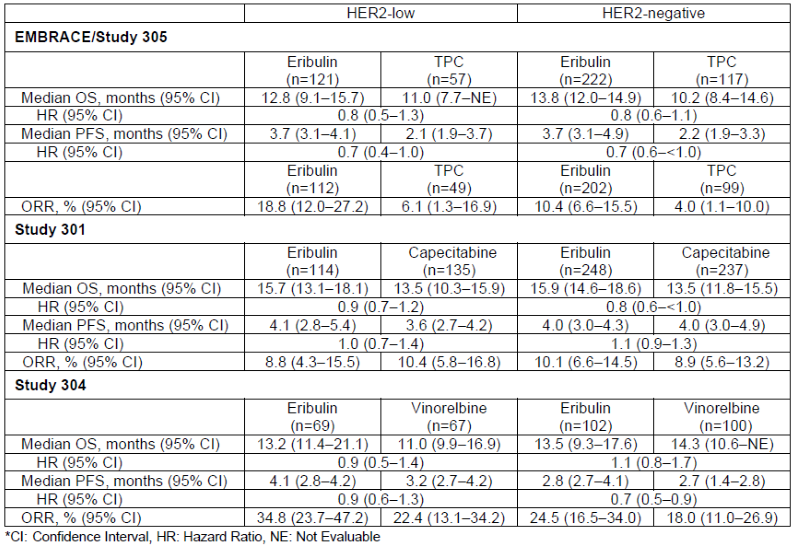
Data from the Post Hoc Analysis
The post hoc analysis included data from three trials — eribulin vs. TPC (NCT00388726, EMBRACE trial/Study 305), eribulin vs. capecitabine (NCT00337103, Study 301), and eribulin vs. vinorelbine (NCT02225470, Study 304) in patients with locally recurrent or mBC who had prior lines of chemotherapy treatments (≤2 for Study 301; 2-5 for Study 304 and EMBRACE Trial/Study 305) including an anthracycline and a taxane. A total of 1,589 eligible patients were enrolled in the EMBRACE trial/Study 305, Study 301 and Study 304, and baseline characteristics were generally balanced between treatment arms in all studies.
Median overall survival (OS), median progression free survival (PFS) and objective response rate (ORR) were analyzed. PFS and ORR were measured per Response Evaluation Criteria in Solid Tumors Version (RECIST) (v1.0 for EMBRACE trial/Study 305 and Study 301; v1.1 for Study 304) by independent imaging review. ORR was measured in evaluable patients (EMBRACE trial/Study 305) and in the intent-to-treat population (Study 301 and Study 304).
In the post hoc analysis, OS, PFS, and ORR among patients with HER2-low or HER2-negative status were generally similar to those of the eribulin treatment arms overall in each of the EMBRACE trial/Study 305, Study 301 and Study 304.3,4,5 Efficacy results for patients with HER2-low and HER2-negative status across all three studies are summarized in the table below:
Media Inquiries:
Public Relations Department,
Eisai Co., Ltd.
+81-(0)3-3817-5120
[Notes to editors]
1. About Metastatic Breast Cancer
Metastatic breast cancer (mBC) is an advanced stage of the disease that occurs when cancer spreads beyond the breast to other parts of the body. It is estimated there were more than 2,261,000 new cases of breast cancer and more than 684,000 deaths from the disease globally in 2020.6 In Japan, it is estimated there were more than 92,000 new cases of breast cancer diagnosed and more than 17,000 deaths from this disease in 2020.7 In the United States, it is estimated that approximately 288,000 women will be diagnosed with breast cancer and over 43,000 women will die from the disease in 2022.1 It is estimated that 30% of people with early-stage breast cancers will go on to develop metastatic disease,8 and approximately 6% of women with breast cancer will have metastatic disease at the time of diagnosis. Metastatic breast cancer has a poor prognosis compared to non-metastatic breast cancer.9 The estimated 5-year relative survival rate for women with mBC is 28%.10
2. About eribulin mesylate (product name: Halaven, “eribulin”)
Eribulin is a microtubule dynamics inhibitor in the halichondrin class with a novel mechanism of action, developed in-house by Eisai. Structurally, eribulin is a simplified and synthetically produced version of halichondrin B, a natural product isolated from the marine sponge Halichondria okadai. Eribulin is believed to work by inhibiting the growth phase of microtubule dynamics which prevents cell division. In addition, non-clinical studies showed eribulin’s unique actions in the tumor microenvironment such as an increase in vascular perfusion and permeability in tumor cores,11 promotion of the epithelial state, decrease in capacity of breast cancer cells to migrate,12 etc.
Eribulin has been approved for the indications below.
Breast cancer (Approved in over 80 countries Japan, the United States, China, and countries in Europe and Asia)
Japan: Inoperable or recurrent breast cancer
The United States: The treatment for patients with metastatic breast cancer who have received at least two chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Europe: The treatment of adult patients with locally advanced or metastatic breast cancer who have progressed after at least one chemotherapeutic regimen for advanced disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting unless patients were not suitable for these treatments.
Sarcoma (Approved in over 80 countries Japan, the United States, and countries in Europe and Asia)
Japan: Soft tissue sarcoma
The United States: The treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen
Europe: The treatment of adult patients with unresectable liposarcomas who have received prior anthracycline containing therapy (unless unsuitable) for advanced or metastatic disease
1 National Institutes of Health, National Cancer Institute website, “Cancer Stat Facts: Female Breast Cancer”:
https://seer.cancer.gov/statfacts/html/breast.html
2 U.S. FOOD & DRUG ADMINISTRATION website, “FDA Approves First Targeted Therapy for HER2-Low Breast Cancer”:
3 Cortés J et al. Lancet. 2011 Mar 12;377(9769):914-23.
https://www.thelancet.com/action/showPdf?pii=S0140-6736%2811%2960070-6
4 Kaufman PA et al. J Clin Oncol. 2015 Feb 20;33(6):594-601.
https://ascopubs.org/doi/pdfdirect/10.1200/JCO.2013.52.4892
5 Yuan P et al. Eur J Cancer. 2019;112:57-65.
https://www.ejcancer.com/article/S0959-8049(19)30139-X/pdf
6 International Agency for Research on Cancer, World Health Organization. “Breast Fact Sheet.” Cancer Today, 2020.:
https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf
7 International Agency for Research on Cancer, World Health Organization. “Japan Fact Sheet.” Cancer Today, 2020.:
https://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-sheets.pdf
8 BREASTCANCER ORG website, “Metastatic Breast Cancer”:
https://www.breastcancer.org/types/metastatic
9 American Cancer Society. “Breast Cancer Facts & Figures 2019-2020”:
10 Cancet.Net website. “Breast cancer – Metastatic: Statistics”:
https://www.cancer.net/cancer-types/breast-cancer-metastatic/statistics
11 Funahashi Y et al., Cancer Sci., 2014; 105, 1334-1342
https://onlinelibrary.wiley.com/doi/epdf/10.1111/cas.12488
12 Yoshida T et al., Br J Cancer, 2014; 110, 1497-1505
https://www.nature.com/articles/bjc201480
